Twenty-twelve was a big year for ichthyosaurology. For me, it was my first full academic and calendar years as a PhD student at the University of Bristol studying ichthyosaurs. It also marks the 15th month of this blog’s existence and this will be the 35th post. That averages to more than one each month, which pleases me as there have been many occasions when I didn’t feel that I’ve lived up to my promises. Not that this means that I have.
So what about now? As this will be one of the first posts of 2013, why not cover some of my, and (mostly) others, work from the past year, as well as my plans for the next. Prepare to be dazzled!
Ichthyosaurs in 2012
The so-called ‘ichthyosaur renaissance’ is continuing in full swing with many people working on many exciting things. Last year saw new specimens unearthed and described, and at least five new species and three new genera named officially: Acamptonectes densus (Fischer et al. 2012), Stenopterygius aaleniensis (Maxwell, Fernández and Schoch 2012), Temnodontosaurus azerguensis (Martin et al. 2012), Cryopterygius kristiansenae and Palvennia hoybergeti (Druckenmiller et al. 2012). From all the research that came out last year, here’s a few pieces on some of the findings.
Stenopterygius galore
January was a bumper month, and great way to start the year. Erin Maxwell (2012a, b) published studies on the genus Stenopterygius from the Lower Jurassic Posidonienschiefer (Posidonia Shale) of southwestern Germany. Stenopterygius is certainly the most numerous and probably the most completely known ichthyosaur. Huge amounts of material have been found of this genus and now fill collections in the Museum für Naturkunde, Stuttgart, Urwelt-Museum Hauff in Holzmaden and many others besides. Examples can be seen in Heinrich Mallison’s recent post from his Palaeontology of SW Germany series. Having such an excellent record lends itself to detailed studies in the biology of Stenopterygius.
Species level metrics

Figure 1. Morphospace plot of Stenopterygius from the Posidonienschiefer. This shows the distributions of three species: S. quadriscissus, S. triscissus and S. uniter. Component 1 is related to size and growth. The starred specimens are embryos (to the left) and their mothers (to the right). Circled points are type specimens. (From Maxwell 2012a.)
Having so much material available frequently leads to an excess of enthusiasm in naming species, as has happened so often in ichthyosaurs. Maisch (2008) cleaned up the taxonomy of Stenopterygius into three species: S. quadriscissus, S. triscissus and S. uniter along with the erection of Hauffiopteryx typicus. The differences between these species are there, but are often subtle and depend frequently on the preservation – as in so many fossils. Using morphometrics – statistical variation in shape – Maxwell (2012a) showed that each species clearly occupies their own separate morphospace: each species is morphologically different (fig. 1). The area each species occupies on this graph also shows the variation within the species. S. quadriscissus is by far the most common and, as expected, shows the greatest variation, whereas S. triscissus and S. uniter don’t show as much. The effect of growth from juveniles to adults can also be seen: the four stars in the S. quadriscissus space mark two specimens of associated adults and juveniles. The large difference along the x-axis shows that size (mostly body length) dominates this variation, but that other changes (y-axis and other components) change noticeably too.
Aalenian treat
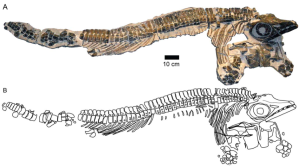
Figure 2. Stenopterygius aaleniensis from the Aalenian of southwestern Germany. this shows the unusual preservation where the body is largely seen side-on, but the head and shoulders are face-on. A is the type specimen and B an interpretative drawing. (From Maxwell, Fernández and Schoch 2012.)
The Middle Jurassic (Aalenian–Callovian; 174–163 Ma) is largely devoid of ichthyosaur fossils worldwide. The Late Callovian Oxford Clay formation is full of Ophthalmosaurus and ichthyosaurs from the Bajocian–Bathonian (170–166 Ma) of South America are known (Fernández 1994, 1999). Stenopterygius aaleniensis (the ‘Aalenian Stenopterygius’) (Maxwell, Fernández and Schoch 2012) is the first conclusive remains from the Aalenian (174–168 Ma), not just of an ichthyosaur, but also of a marine reptile generally (i.e. plesiosaurs, pliosaurs and crocodiles too). This newly described specimen (fig. 2) is found in a similar area to those from the Posidonienschiefer, albeit in higher strata. The three-dimensional specimen has its head stuck out at a completely different angle to the rest of its body, giving a good all-around view; very important in ichthyosaur taxonomy, but made it difficult to incorporate in the two-dimensional morphometric study of the Posidonienschiefer Stenopterygius (Maxwell 2012a).
Cretaceous ichthyosaurs … “the Empire strikes back?”
That is the second part of the brilliantly titled article by Zammit (2012) looking at the diversity of ichthyosaurs in the Cretaceous. For a long time, there was only one genus – Platypterygius – to which almost all Cretaceous ichthyosaurs were assigned. Platypterygius currently contains seven or eight species because of this, although more have come and gone in the past. This is no longer the case: Platypterygius still dominates the diversity, and is in need of the same type of revision that Stenopterygius went through recently (Maisch 2008). However, now there are numerous other genera to accompany it.
This process began relatively recently, with the finding of Caypullisaurus bonapartei (Fernández 2001, 2007) and more recent reports have increased that with Maiaspondylus lindoei (Maxwell and Caldwell 2006b), Athabascasaurus bitumineus (Druckenmiller and Maxwell 2010), Aegirosaurus leptospondylus? (Fischer et al. 2011a), Sveltonectes insolitus (Fischer et al. 2011b) and Acamptonectes densus (Fischer et al. 2012). Along the way material and descriptions of Platypterygius have also been reviewed and revised (e.g. Fernández and Aguirre-Urreta 2005; Maxwell and Caldwell 2006a; Kolb and Sander 2009; Maxwell and Kear 2010; Adams and Fiorillo 2011; Fischer 2011; Pardo-Pérez et al. 2012).
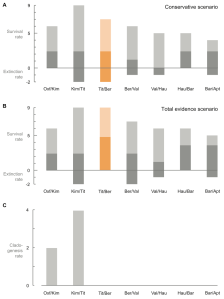
Figure 3. Extinction rates and clade generation rates of ophthalmosaurids (an ichthyosaur subgroup) through the Late Jurassic and Early Cretaceous. The low extinction rates show continuation of taxa across the Jurassic/Cretaceous boundary (orange). In this data there are no new clades (groups) arising after the Late Jurassic. (From Fischer et al. 2012.)
Fischer et al. (2012) discussed the effect of this more recently realised diversity. Their elegantly simple graphs (fig. 3) show that there is low to no extinction in ichthyosaur species over the Jurassic–Cretaceous boundary (145 Ma). It’s also apparent that no new clades arose following the Kimmeridgian/Tithonian either. This implies one or two scenarios:
- Late Jurassic and Cretaceous ichthyosaur lineages had extremely long lifespans.
- The lineage and diversity data is incomplete, either due to incorrect assignment of species (lumping many together) or through incomplete sampling (no one’s bothered to look for any).
The latter of these is mentioned in the body text (Fischer et al. 2012, p. 22), with particular reference to the ‘wastebasket’ nature of Platypterygius and the poor rock record of the earliest Cretaceous. However, the conclusion remains: ichthyosaurs show stability in their diversity over the Jurassic–Cretaceous boundary and through the earliest Cretaceous. The age-old question of why they became extinct towards the end of the Cenomanian (94 Ma) stands.
A few new specimens
Along with Stenopterygius aaleniensis mentioned above, a few more ichthyosaurs took their turn in the spotlight. These add to our knowledge about the occurrences and biology these groups and the ecology they belonged to.
Lower Jurassic

Figure 4. A new specimen of Ichthyosaurus communis from the Pliensbachian of Charmouth. this is the youngest I. communis known and also shows gut contents. (From Bennett et al. 2012.)
Ichthyosaurus communis finds are common along the coast between Lyme Regis and Charmouth (Dorset, UK); at least common for ichthyosaur finds. The preservation of the material can be spectacular – close to that seen in the Posidonienschiefer – but all too often it falls short. A specimen described by Bennett et al. (2012) does look a bit of a mess (fig. 4), but it’s still an informative mess! This specimen contains enough detail to be assigned to I. communis, making this the youngest definite example of that taxon. It was found in Pliensbachian age (191–183 Ma) rocks, whereas I. communis was previously known only from the Rhaetian–Sinemurian ages (208–191 Ma). There are also a number of fish remains preserved in the gullet region giving a glimpse of this ichthyosaur’s lifestyle.

Figure 5. Temnodontosaurus azerguensis from the Toarcian of France. This large ichthyosaur is odd as there are no teeth associated with it, other Temnodontosaurus are well known for having big teeth. (From Martin et al. 2012.)
The new species Temnodontosaurus azerguensis was named based on material from the Toarcian (183–174 Ma) of France (Martin et al. 2012). Again, this is another impressive specimen, for its preservation and its size, at the best part of 8 m long (fig. 5). The Toarcian is the age in which the Posidonienschiefer was deposited, making this specimen about, but slightly younger than the – three of the four – Stenopterygius discussed above. As Temnodontosaurus go, T. azerguensis is weird – which seems to be the fad for new ichthyosaurs nowadays: weird is good. There were no teeth found with the specimen and the snout is long and thin and with reduced dental grooves. This compares with the “large size … and massive dentition” (Martin et al. 2012, p. 1002) usually associated with Temnodontosaurus. Being from later in the Toarcian also means this ichthyosaur is from after the Toarcian Oceanic Anoxic Event. This ‘stagnation’ of the ocean occurs as oxygen levels in the ocean fall; fish and invertebrates then die and there is a bloom in decay bacteria, which then causes a further reduction in oxygen levels. An event like this leaves its trace in black, organic-rich strata, like the Posidonienschiefer. Few ichthyosaur remains are known from this time; whether this new species marks a post-event radiation cannot be determined.

Figure 6. An embryo of Leptonectes from Somerset. Young are often identifiable by their large eyes. Several of the embryo’s bones are scattered, and there are vertebrae from the associated adult too. (From Lomax and Massare 2012.)

Figure 7. A famous Stenopterygius specimen. This is usually interpreted as an adult with a juvenile in the process of being birthed: ichthyosaurs gave birth to live young, as with modern mammals. It is this, among other things, that likely allowed them to become so highly adapted to their marine lifestye. (From Organ et al. 2009.)
While it may not be a new species, coverage of a Leptonectes with associated embryos is still exciting (Lomax and Massare 2012) (fig. 6). This was much closer to (my) home, being found only in Street (Somerset, UK), however the specimen is housed in the Sedgwick Museum, Cambridge; a little further a travel. Embryos have been known in ichthyosaurs for a long time (see this famous Stenopterygius (fig. 7)), but this is the first occurrence in Leptonectes.
Upper Jurassic
The Upper Jurassic had a boost in attention towards the end of this year. I’ve covered the discovery of Cryopterygius kristiansenae and Palvennia hoybergeti in a post last year (Druckenmiller et al. 2012; ‘In the land of Svalbard…’). Also published was a description of some new material of Arthropterygius sp. from Argentina. Curiously, Arthropterygius was only previously known from Canada (Maxwell 2010) – several thousand miles away today, and not much less in the Jurassic. As shocking as this may seem, it isn’t unheard of: examples of Ophthalmosaurus are known the (western) world over; from England (Seeley 1874; Kirton 1983), Mexico (Buchy 2010) and Argentina (as Ancanamunia; Rusconi 1948; Kirton 1983; Fernández and Maxwell 2012). Modern Grey Whales also travel great distances – from their tropical birthing grounds towards the Arctic for feeding.
Palaeobiology

Figure 8. The effects of ‘the bends’ can be seen in the bones of this Leptonectes tenuirostris. The bone cells are killed, and the stricture weakened, which causes failure when the bone is loaded. the shaft of the arrow is 40 mm. (From Rothschild, Xiaoting and Martin 2012.)
Besides naming new ichthyosaurs, a few papers discussed the palaeobiology of these animals both during life and after death. Rothschild, Xiaoting and Martin (2012) looked at decompression syndrome (‘the bends’) in ichthyosaurs from the Triassic in comparison to post-Triassic. Evidence for the bends can be seen in the state of the bones of ichthyosaurs: build-up of nitrogen in the bones blocking blood vessels and killing cells causes deformities (fig. 8). The findings show that Triassic ichthyosaurs – along with Stenopterygius – apparently lived a more sedate life than most of their Jurassic and Cretaceous relatives (see Rothschild, Xiaoting and Martin 2012, tbl. 1). The authors suggest several reasons for this increase in the bends in later ichthyosaurs:
- Later ichthyosaurs, particularly the thunniform (tuna-shaped) group are more adapted to deep-diving so will be more affected by repeated decompression as they surface to breath (Motani, Rothschild and Wahl 1999).
- Triassic ichthyosaurs dominated the top tiers of the food web whereas Jurassic and Cretaceous ichthyosaurs faced more predation from sharks, pliosaurs and marine crocodiles (Fröbisch et al. 2013).
- Fast-swimming teleost fish evolved in the Late Jurassic and Early Cretaceous, which may have caused more accidental decompression in hunting. This may be associated with a more endothermic (‘warm-blooded’) metabolism (Bernard et al. 2010).
Incidentally, it was this article, along with its comments and replies (Hayman 2012; Rothschild 2012) that led to John Tennant at Green Tea and Velociraptors to write a letter.
Nakajima, Houssaye and Endo (2012, accepted and published online) gave more insights on early ichthyosaur biology. This study on Utatsusaurus hataii – one of the earliest and most basal ichthyopterygians – looked at the bone structure in the ribs and humeri of two specimens. The results suggest rapid growth and possibly raised metabolic rates: early in their evolution – and so soon after the Permo-Triassic extinction event (within 6–8 Ma) – these ichthyosaurs were already developing the characters that made them successful for 160 Ma. That Utatsusaurus had paddles and a long, finned tail was already known. The high growth and metabolic rates, along with spongy inner bone structure, hints at a pelagic (open ocean) lifestyle and palaeoecology.

Figure 9. The scattered remains of a Stenopterygius from the Posidonienschiefer. Specimens with the bones spread like this are not uncommon. The diffuse substrate and even low-velocity currents are the likely cause of this preservation. (From Reisdorf et al. 2012.)
To conclude the past year in ichthyosaur research, a look at their taphonomy. This takes us back, as has happened so often this year, to the Posidonienschiefer and exploding carcasses (Reisdorf et al. 2012). In recent years, the phenomenon of exploding whale carcasses has gained several hits, particularly from Inside Natures’ Giants and on YouTube (vid. 1). These incidences have led to the postulation that some of the scattered ichthyosaur remains found could be due to this cause (e.g. Böttcher 1989; Martill 1993; fig. 9). Explosions of carcasses in modern whales tends only to happen when there’s something around to disturb the carcass – frequently a person with a harpoon (vid. 1, fig. 10).

Figure 10. Stills from the above video showing the effect of sticking a pointed stick into a bloated whale. (From Reisdorf et al. 2012.)
The carcass itself will tend to sink and – in deep enough water – the pressure will prevent explosion. The alternative mechanism Reisdorf et al. (2012) suggest is the sediments and currents. Slight currents would have been free to move the bones around in the nebulous ‘soupy substrates’ that made up the seabed (Martill 1993). Velocities of only 0.2–0.4 m/s (<1 mph; a very slow walking pace) would’ve be enough to move around the flank and belly ribs and the paddle bones causing such preservation.
Conclusions
This concludes my brief (really?) look at the published ichthyosaur research from 2012, but has not been comprehensive for the amount of research, or the summaries themselves. Twenty-thirteen has already seen ichthyosaur articles published (e.g. Fröbisch et al. 2013; Maxwell and Dececchi 2013), preview manuscripts (e.g. Nakajima, Houssaye and Endo 2012) and more that are currently in preparation.
I know what I did last year
From other peoples’ work to what I’ve been doing. As I began with, 2012 was my first full (calendar) year in my PhD and a year later I feel I’ve made progress: I’m almost getting to the results stage! My original plans (here, here and here) have changed somewhat due to feasibility, time and material constraints, but the gist remains the same.
Taxonomy
The largest part of this year has been taken up with describing those ichthyosaurs from the Middle and Upper Jurassic. Fortunately, I was awarded several grants from the Palaeontographical Society, the Geological Society of London, the Systematics Association and the Bob Savage Memorial Fund. This money was spent between August and today on visits to various museums. My visit to Leicester received its own post, but I also went to the Hunterian Museum, Glasgow, Sedgwick Museum, Cambridge, University Museum of Natural History, Oxford and the Natural History Museum, London. I still have to visit Peterborough Museum in the coming weeks.

Figure 11. Ophthalmosaurus icenicus in the main hall at the Natural History Museum, London. This specimen is about 5 m long.
All this travelling was, for the most part, in search of as much of the material of Ophthalmosaurus from the Leeds Collections that I could find. I’ve only mentioned these collections briefly and they deserve to have more detail on them: one of my tasks for this year. All this travelling led me to over 300 specimens referred to Ophthalmosaurus, covering every (known) bone in its body (fig. 11).

Figure 12. ‘Grendelius’ mordax on display in the Sedgwick Museum, Cambridge. the lower jaw is about 1.2 m long.
In some select places, particularly the Natural History and Sedgwick museums, there were those more elusive treats Brachypterygius and Nannopterygius. In Cambridge, I came across the giant skull originally named Grendelius (fig.) and London has the difficult to see skeleton of Nannopterygius (fig.) This last is still causing me access problems due to its position (~6 m up on a wall), but this should hopefully be resolved later this year.

Figure 13. Nannopterygius enthekiodon, the best photo I could in the Natural History Museum, London. The specimen is about 3 m long.
The descriptions of these three ichthyosaurs are largely complete in draft form. The basis was taken from Kirton (1983), as I’ve mentioned before, and I’ve checked and added much to it. Much that now needs to be done revolves around editing this into a manuscript, and writing the introductory and discussion sections.
Phylogeny
When I haven’t been doing stuff with ichthyosaurs descriptions, I’ve been working on their relationships. This is continuing as originally planned, and I now have a set of characters and the first stages of a matrix, which is progressing well. The collection, comparing and construction of this has been time consuming because it requires detailed qualitative understanding of the ichthyosaurs themselves (all 101 species, and counting) and the characters themselves. Because the characters I’ve collected are derived from several sources, their purposes and nature can be very different. These wrinkles have to be resolved before any analysis can take place; this is where I am now.
Other odd jobs
Much of the studies that I wrote about in ‘My PhD: Part 3’ require a complete, robust phylogeny; hence have not been properly started yet. It is also here that some of the greatest changes have happened. I had hoped to use some three-dimensionally preserved ichthyosaur skulls (e.g. those from Caine and Benton 2011), CT scanned, as the basis for a finite element analysis of their jaw mechanics. Unfortunately, due to the complexity of the skulls (50+ elements), the problems of modelling sutures (where these elements meet) and it taking a long time to build these models, it doesn’t seem feasible to do this (yet).

Figure 14. Preliminary fluid dynamics testing. The silhouette is of Temnodontosaurus from Motani (2005).
Instead, following the suggestion of one of my supervisors, Emily Rayfield, I’ve been looking at the related modelling of fluids (computational fluid dynamics). The idea with this will be to use two-dimensional body silhouettes to look at the efficiency of ichthyosaurs’ motion through the water. This is very much in the designing/planning/guessing stage. I’ve run some preliminary simulations (fig. 14) just to look at how, and if, the model works. Next will be to compare with flume experiments (e.g. Ferry and Lauder 1996) to make the results are realistic. Eventually this can be combined with the phylogeny to see if there are any trends through ichthyosaur evolution.
Everything else
Outside of my research, 2012 offered the chance to go to several meetings:
- Big Palaeontology was this year’s Lyell Meeting at the Geological Society (29 March), featuring talks on topics ranging from specimen databases and virtual palaeontology through to fossil code and palaeontology of the Jurassic Coast World Heritage Site.
- The Symposium of Vertebrate Palaeontology and Comparative Anatomy was held in Oxford (10–15 September), but unfortunately I could only spend a day there.
Besides meetings, I also gave a few talks (sorry, these were on my research):
- 29 November: Large-scale evolutionary trends in ichthyosaurs, departmental presentation; extended for the West of England Geologists’ Association on 15 January 2013.
Full ahead Cap’n
So what does the year ahead hold for me? Currently I’ve spent the best part of the last month not writing this blog post, and the last two days writing all 3000+ words of it, which has been good for affirming my ability to write (the amount of editing and making images notwithstanding). As last year had much work on the descriptions of Ophthalmosaurus, Brachypterygius and Nannopterygius, this month I’ve largely put that aside, until after visiting Peterborough. On the other hand, I am in the process of writing a proposal for a Society of Vertebrate Paleontology Memoir I’ve looked at many places to publish, with the criteria of quality, circulation and accessibility (not in that order). PLOS ONE looked promising (thanks to Darren Naish for the suggestion), but unfortunately they just won’t accept monographs (large, complete bodies of work, which this will be). The SVP memoir series offered the best mix of these three criteria; should it be accepted, it will come out in 2014.
I have however picked up on creation the character list and matrix for my phylogeny, looking to complete this within ~two months. So many other things will be born from this and take up so much of the rest of my year – a flurry of projects, as I like to think of it.
With all this work going on and (hopefully) being completed, 2013 is looking like a busy and rewarding year. I am expecting to have my first peer-reviewed publications, first conferences talk(s) and poster(s) and be largely on the way to completing my PhD.
Three years? I can do it in that!
Roll on 2013.
References
HAYMAN, J. 2012. Deep-diving dinosaurs. Naturwissenschaften, 99, 671–672.
KIRTON, A. M. 1983. A review of British Upper Jurassic ichthyosaurs. Unpublished PhD Thesis, University of Newcastle-upon-Tyne, Newcastle-upon-Tyne, 239 pp., 45 figs., 5 pls.
KOLB, C. and SANDER, P. M. 2009. Redescription of the ichthyosaur Platypterygius hercynicus (Kuhn 1946) from the Lower Cretaceous of Salzgitter (Lower Saxony, Germany). Palaeontographica Abteilung A: Paläozoologie—Stratigraphie, 288, 151–192.
LOMAX, D. R. and MASSARE, J. A. 2012. The first reported Leptonectes (Reptilia: Ichthyosauria) with associated embryos, from Somerset, England. Paludicola, 8, 263–276.
MAISCH, M. W. 2008. Revision der Gattung Stenopterygius Jaekel, 1904 emend. von Huene, 1922 (Reptilia: Ichthyosauria) aus dem unteren Jura Westeuropas. Palaeodiversity, 1, 227–271. [In German.] 🔓
MARTILL, D. M. 1993. Soupy substrates: a medium for the exceptional preservation of ichthyosaurs of the Posidonia Shale (Lower Jurassic) of Germany. Kaupia, 2, 77–97.
—— ROTHSCHILD, B. M. and WAHL, W. R. 1999. Large eyeballs in diving ichthyosaurs. Nature, 202, 747.
RUSCONI, C. 1948. Ictiosaurios del Jurásico de Mendoza. Revista del Museo de Historia Natural de Mendoza, 2, 17–162. [In Spanish.]

